Parameter Set Anti-HIV Drugs - LC-MS/MS
Encompasses 17 analytes
6PLUS1® Multilevel Calibrator Set available
Part of the MassTox® TDM Series A
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
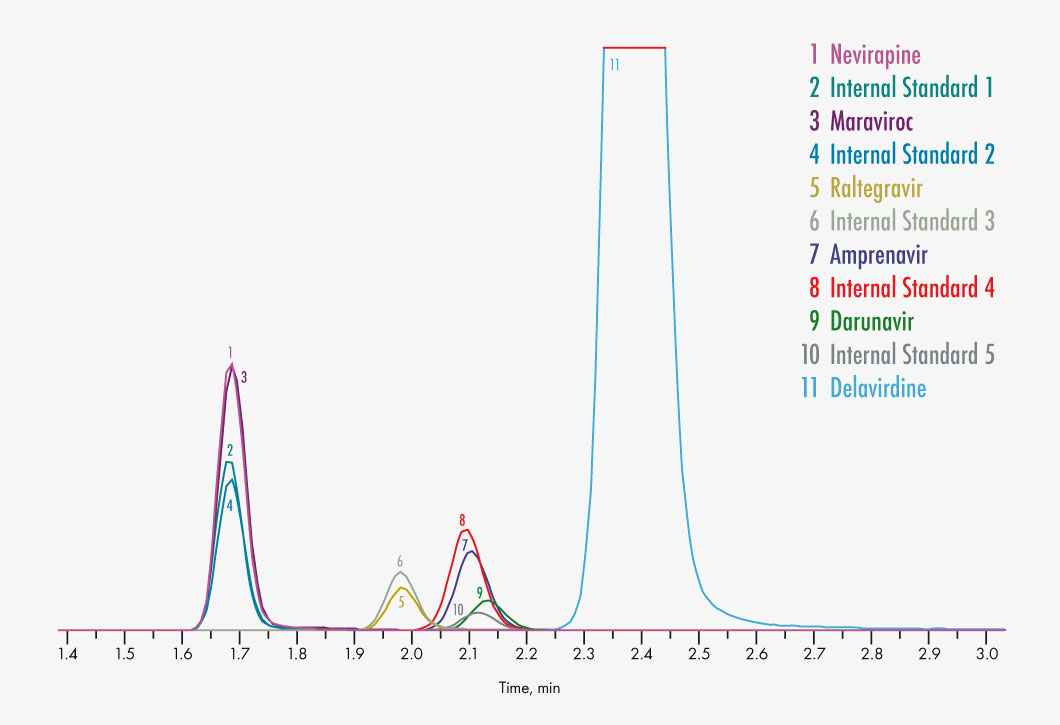

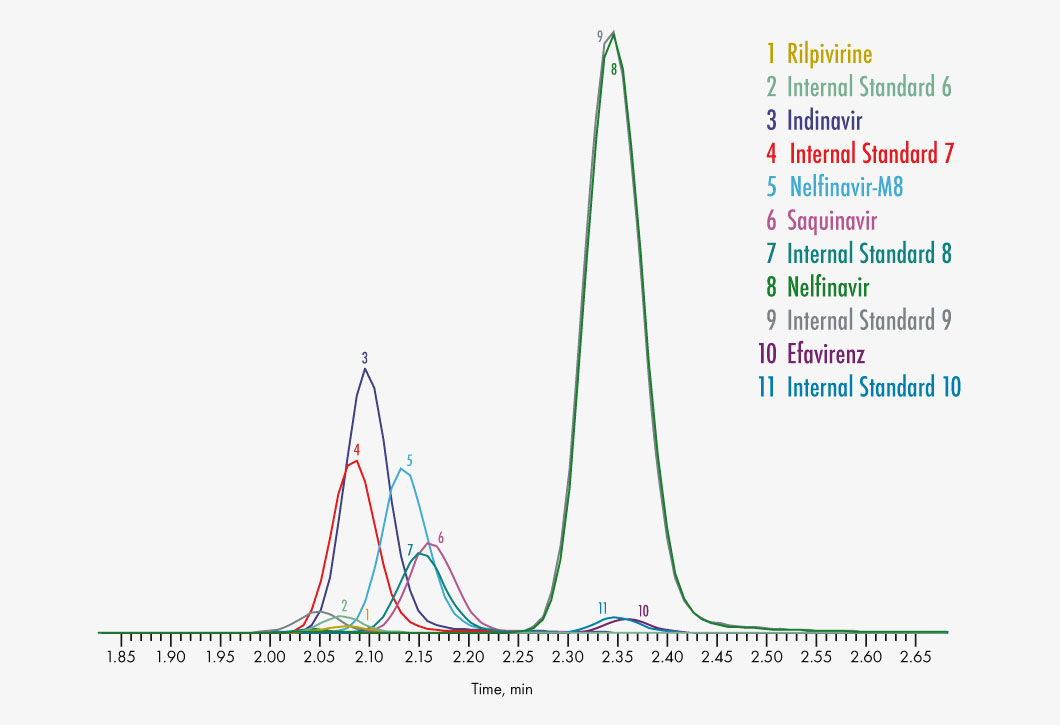

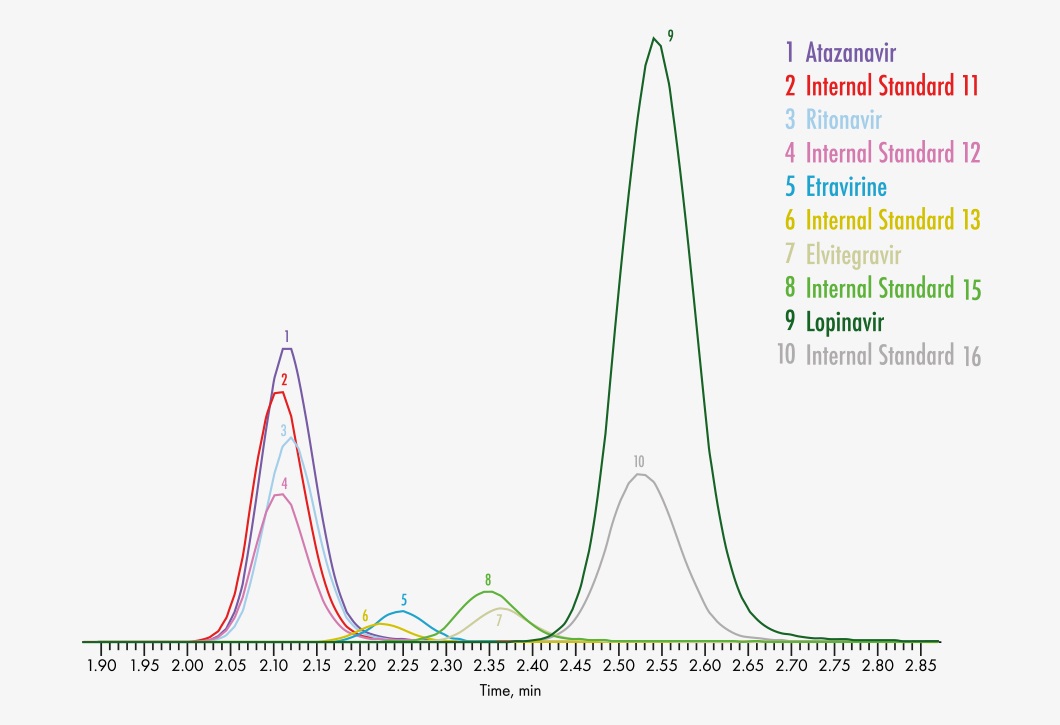

Amprenavir
Atazanavir
Darunavir
Delavirdine
Efavirenz
Elvitegravir
Etravirine
Indinavir
Lopinavir
Maraviroc
Nelfinavir
Nelfinavir-M8
Nevirapine
Raltegravir
Rilpivirine
Ritonavir
Saquinavir
Clinical relevance
According to estimates from UNAIDS there are about 36.9 million people worldwide living with HIV, of which approximately 3 million are children under the age of 15 years. Even if the number of globally registered new infections is decreasing, it was still at approx. 2 million in 2014. The currently available drugs, which are usually prescribed as part of HAART (highly active antiretroviral therapy), interfere with or prevent the development of the virus at different stages of reproduction or cell infection.
Regular monitoring of drug concentration is very important, especially in HIV therapy. Sufficiently high levels of the administered antiretroviral drugs in plasma are a key factor in the success of the treatment. Individual plasma levels can fluctuate significantly and low levels can affect the success of the treatment. On the other hand high concentrations can cause major side effects, such as disorders of the central nervous system, liver toxicity, gastrointestinal disorders or renal toxicity. Therefore the measurement of drug concentrations in serum or plasma is essential for optimising the therapy.
Product advantages
With the Parameter Set Anti-HIV Drugs in serum/plasma, 18 different drugs can be measured quickly and efficiently using LC-MS/MS. Due to the careful optimisation of all kit components as well as the chromatographic separation, matrix effects (“ion suppression”) are minimised and the robustness of the method is enhanced. Sample preparation is based on a protein precipitation step. The use of stable isotopically labelled (deuterated) and co-eluting internal standards as well as 6PLUS1® multilevel calibrators ensures high precision and the reproducible and reliable quantification of the analytes. The method is comprehensively validated.
The Parameter Set is a part of the Series A modular system, which enables the analysis of all parameters without switching column or changing the mobile phases, thereby minimising required workload in the laboratory. The Basic Kit A contains all components required for sample preparation and all necessary mobile phases. The MasterColumn® A is the analytical column used for the determination of all Series A analytes. Our portfolio contains further MassTox® Parameter Sets.
For the analysis you require the MassTox® TDM Basic Kit A, the specific MassTox® TDM Parameter Set and the analytical column MassTox® TDM MasterColumn® A.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 1.0 – 65 µg/l |
| Upper Limit of Quantification | up to 168 mg/l |
| Intraassay | CV= 1.0 – 6.7 % |
| Interassay | CV= 1.5 – 8.1 % |
| Recovery | 88 – 111 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3.0 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Amprenavir, Atazanavir, Darunavir, Efavirenz, Elvitegravir, Etravirine, Indinavir, Lopinavir, Maraviroc, Nelfinavir, Nelfinavir-M8, Raltegravir, Ritonavir, Saquinavir |
-
 Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately
Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately -
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS
Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS






Amprenavir
Atazanavir
Darunavir
Delavirdine
Efavirenz
Elvitegravir
Etravirine
Indinavir
Lopinavir
Maraviroc
Nelfinavir
Nelfinavir-M8
Nevirapine
Raltegravir
Rilpivirine
Ritonavir
Saquinavir
Clinical relevance
According to estimates from UNAIDS there are about 36.9 million people worldwide living with HIV, of which approximately 3 million are children under the age of 15 years. Even if the number of globally registered new infections is decreasing, it was still at approx. 2 million in 2014. The currently available drugs, which are usually prescribed as part of HAART (highly active antiretroviral therapy), interfere with or prevent the development of the virus at different stages of reproduction or cell infection.
Regular monitoring of drug concentration is very important, especially in HIV therapy. Sufficiently high levels of the administered antiretroviral drugs in plasma are a key factor in the success of the treatment. Individual plasma levels can fluctuate significantly and low levels can affect the success of the treatment. On the other hand high concentrations can cause major side effects, such as disorders of the central nervous system, liver toxicity, gastrointestinal disorders or renal toxicity. Therefore the measurement of drug concentrations in serum or plasma is essential for optimising the therapy.
Product advantages
With the Parameter Set Anti-HIV Drugs in serum/plasma, 18 different drugs can be measured quickly and efficiently using LC-MS/MS. Due to the careful optimisation of all kit components as well as the chromatographic separation, matrix effects (“ion suppression”) are minimised and the robustness of the method is enhanced. Sample preparation is based on a protein precipitation step. The use of stable isotopically labelled (deuterated) and co-eluting internal standards as well as 6PLUS1® multilevel calibrators ensures high precision and the reproducible and reliable quantification of the analytes. The method is comprehensively validated.
The Parameter Set is a part of the Series A modular system, which enables the analysis of all parameters without switching column or changing the mobile phases, thereby minimising required workload in the laboratory. The Basic Kit A contains all components required for sample preparation and all necessary mobile phases. The MasterColumn® A is the analytical column used for the determination of all Series A analytes. Our portfolio contains further MassTox® Parameter Sets.
For the analysis you require the MassTox® TDM Basic Kit A, the specific MassTox® TDM Parameter Set and the analytical column MassTox® TDM MasterColumn® A.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 1.0 – 65 µg/l |
| Upper Limit of Quantification | up to 168 mg/l |
| Intraassay | CV= 1.0 – 6.7 % |
| Interassay | CV= 1.5 – 8.1 % |
| Recovery | 88 – 111 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3.0 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Amprenavir, Atazanavir, Darunavir, Efavirenz, Elvitegravir, Etravirine, Indinavir, Lopinavir, Maraviroc, Nelfinavir, Nelfinavir-M8, Raltegravir, Ritonavir, Saquinavir |
-
 Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately
Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately -
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS
Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS