Parameter Set Antidepressants 1/EXTENDED - LC-MS/MS
Encompasses 13 analytes
3PLUS1® Multilevel Calibrator Set available
Part of the MassTox® TDM Series A
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
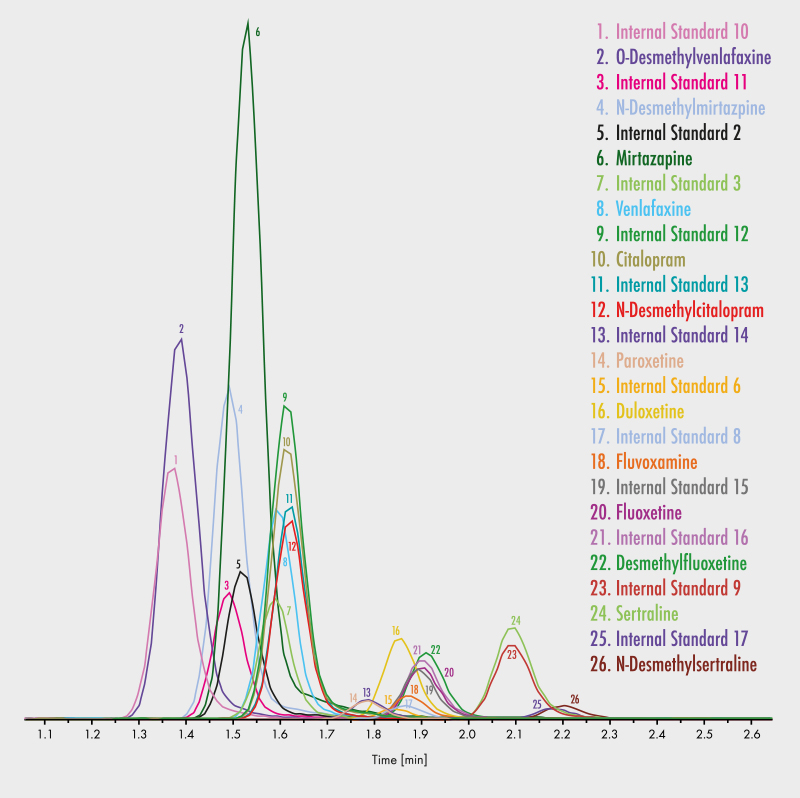

Citalopram
N-Desmethylcitalopram
Duloxetine
Fluoxetine
Desmethylfluoxetine
Fluvoxamine
Mirtazapine
N-Desmethylmirtazapine
Paroxetine
Sertraline
N-Desmethylsertraline
Venlafaxine
O-Desmethylvenlafaxine
Clinical relevance
There are many drugs available for the treatment of depression. In addition to classic tricyclic antidepressant drugs, there are newer generation antidepressants: "selective serotonin re-uptake inhibitors" (SSRIs) and "selective serotonin and noradrenalin re-uptake inhibitors" (SSNRIs).
SSRIs inhibit the re-uptake of the messenger substance serotonin into the presynaptic neurons. They possess characteristics that reduce anxiety and improve mood. Usually, these positive effects start to set in about two to three weeks after starting therapy. SSNRIs are mood and motivation enhancing and act similarly to the SSRIs, but additionally block the re-uptake of noradrenalin.
Since SSRIs and SSNRIs work selectively, side effects are rare. But still, therapeutic drug monitoring is recommended, because the therapeutic endpoint is difficult to determine, and monitoring patient compliance increases the likelihood of treatment success.
Product advantages
With the Parameter Set Antidepressants 1/EXTENDED in serum/plasma, 13 different analytes can be measured fast and efficiently using LC-MS/MS. Due to the careful optimisation of all kit components as well as the chromatographic separation, matrix effects (“ion suppression”) are minimised and the robustness of the method is enhanced. Sample preparation is based on a protein precipitation step. The use of stable isotopically labelled (deuterated) and co-eluting internal standards as well as 3PLUS1® multilevel calibrators ensures high precision and the reproducible and reliable quantification of the analytes. The method is comprehensively validated.
The Parameter Set is a part of the Series A modular system, which enables the analysis of all parameters without switching column or changing the mobile phases, thereby minimising required workload in the laboratory. The Basic Kit A contains all components required for sample preparation and all necessary mobile phases. The MasterColumn® A is the analytical column used for the determination of all Series A analytes. Our portfolio contains further MassTox® Parameter Sets.
For the analysis you require the MassTox® TDM Basic Kit A, the specific MassTox® TDM Parameter Set and the analytical column MassTox® TDM MasterColumn® A.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.5 – 30.0 µg/l |
| Upper Limit of Quantification | at least twice the therapeutic range |
| Intraassay | CV < 9 % |
| Interassay | CV < 9 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3.0 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | 0.00-0.20 min 0 % Mobile Phase 2 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Citalopram, Desmethylfluoxetine, Duloxetine, Fluoxetine, Fluvoxamine, Mirtazapine, N-Desmethylcitalopram, N-Desmethylmirtazapine, N-Desmethylsertraline, O-Desmethylvenlafaxine, Paroxetine, Sertraline, Venlafaxine |
-
 Internal Standard Set Antidepressants 1/EXTENDED and Neuroleptics 1/EXTENDEDOrder no.: 92046/AN1/XT
Internal Standard Set Antidepressants 1/EXTENDED and Neuroleptics 1/EXTENDEDOrder no.: 92046/AN1/XTComponent of the Parameter Sets Antidepressants 1/EXTENDED and Neuroleptics 1/EXTENDED, available separately
-
 3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS -
 MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Antidepressants 1/EXTENDEDOrder no.: 92016/A1/XTTuning Mix for the Parameter Set Antidepressants 1/EXTENDED - LC-MS/MS
Tuning Mix Antidepressants 1/EXTENDEDOrder no.: 92016/A1/XTTuning Mix for the Parameter Set Antidepressants 1/EXTENDED - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
-
 MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS


Citalopram
N-Desmethylcitalopram
Duloxetine
Fluoxetine
Desmethylfluoxetine
Fluvoxamine
Mirtazapine
N-Desmethylmirtazapine
Paroxetine
Sertraline
N-Desmethylsertraline
Venlafaxine
O-Desmethylvenlafaxine
Clinical relevance
There are many drugs available for the treatment of depression. In addition to classic tricyclic antidepressant drugs, there are newer generation antidepressants: "selective serotonin re-uptake inhibitors" (SSRIs) and "selective serotonin and noradrenalin re-uptake inhibitors" (SSNRIs).
SSRIs inhibit the re-uptake of the messenger substance serotonin into the presynaptic neurons. They possess characteristics that reduce anxiety and improve mood. Usually, these positive effects start to set in about two to three weeks after starting therapy. SSNRIs are mood and motivation enhancing and act similarly to the SSRIs, but additionally block the re-uptake of noradrenalin.
Since SSRIs and SSNRIs work selectively, side effects are rare. But still, therapeutic drug monitoring is recommended, because the therapeutic endpoint is difficult to determine, and monitoring patient compliance increases the likelihood of treatment success.
Product advantages
With the Parameter Set Antidepressants 1/EXTENDED in serum/plasma, 13 different analytes can be measured fast and efficiently using LC-MS/MS. Due to the careful optimisation of all kit components as well as the chromatographic separation, matrix effects (“ion suppression”) are minimised and the robustness of the method is enhanced. Sample preparation is based on a protein precipitation step. The use of stable isotopically labelled (deuterated) and co-eluting internal standards as well as 3PLUS1® multilevel calibrators ensures high precision and the reproducible and reliable quantification of the analytes. The method is comprehensively validated.
The Parameter Set is a part of the Series A modular system, which enables the analysis of all parameters without switching column or changing the mobile phases, thereby minimising required workload in the laboratory. The Basic Kit A contains all components required for sample preparation and all necessary mobile phases. The MasterColumn® A is the analytical column used for the determination of all Series A analytes. Our portfolio contains further MassTox® Parameter Sets.
For the analysis you require the MassTox® TDM Basic Kit A, the specific MassTox® TDM Parameter Set and the analytical column MassTox® TDM MasterColumn® A.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.5 – 30.0 µg/l |
| Upper Limit of Quantification | at least twice the therapeutic range |
| Intraassay | CV < 9 % |
| Interassay | CV < 9 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3.0 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | 0.00-0.20 min 0 % Mobile Phase 2 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Citalopram, Desmethylfluoxetine, Duloxetine, Fluoxetine, Fluvoxamine, Mirtazapine, N-Desmethylcitalopram, N-Desmethylmirtazapine, N-Desmethylsertraline, O-Desmethylvenlafaxine, Paroxetine, Sertraline, Venlafaxine |
-
 Internal Standard Set Antidepressants 1/EXTENDED and Neuroleptics 1/EXTENDEDOrder no.: 92046/AN1/XT
Internal Standard Set Antidepressants 1/EXTENDED and Neuroleptics 1/EXTENDEDOrder no.: 92046/AN1/XTComponent of the Parameter Sets Antidepressants 1/EXTENDED and Neuroleptics 1/EXTENDED, available separately
-
 3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS -
 MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Antidepressants 1/EXTENDEDOrder no.: 92016/A1/XTTuning Mix for the Parameter Set Antidepressants 1/EXTENDED - LC-MS/MS
Tuning Mix Antidepressants 1/EXTENDEDOrder no.: 92016/A1/XTTuning Mix for the Parameter Set Antidepressants 1/EXTENDED - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesCE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
-
 3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antidepressants 1/EXTENDEDOrder no.: 92029/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
-
 MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS
MassCheck® Antidepressants 1/EXTENDED Plasma ControlsOrder no.: 0213/XT; 0214/XT; 0215/XTMassTox® TDM Series A Antidepressants 1/EXTENDED in Serum/Plasma – LC-MS/MS






